Glass has been a fascinating material to humankind since it was first made in about 500 BC. At first thought to possess magical properties, glass has come a long way. It is one of the most versatile and oldest materials in the building industry. From its humble beginnings as a window pane in luxury houses of Pompeii to sophisticated structural members in new age buildings, its role in architecture has evolved over the years.
Composition
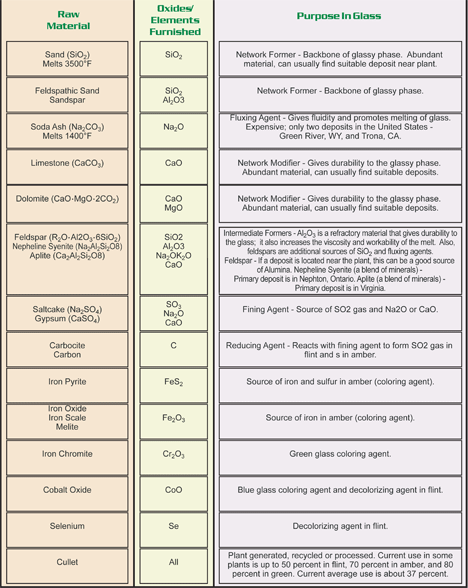
The soda-lime-silicate glass used in buildings has the following composition:
– silica, the raw material, in the form of sand (70 to 72 %)
– soda, the flux, as carbonate and sulphate (approximately 14 %)
– lime, a stabiliser, in the form of limestone (approximately 10 %)
– various other oxides such as alumina and magnesia, to improve the physical properties of the glass, including its resistance to
atmospheric pollutants
– for body-tinted glasses, metal oxides can also be incorporated.
MANUFACTURING OF FLOAT GLASS
While each glass plant is different from the other, the float glass production process can be divided into five universal steps:
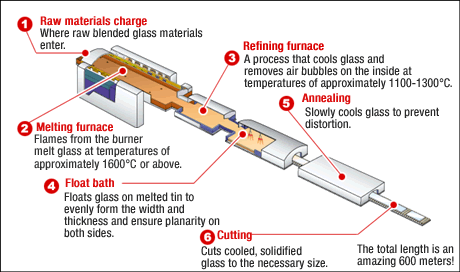
1. Batching of raw materials:

The main components, namely, soda lime glass, silica sand (73%), calcium oxide (9%), soda (13%) and magnesium (4%), are weighed and mixed into batches to which recycled glass (cullet) is added. The use of ‘cullet’ reduces the consumption of natural gas. The materials are tested and stored for later mixing under computerised control.
2. Melting of raw materials in the furnace:
The batched raw materials pass from a mixing silo to a five-chambered furnace where they become molten at a temperature of approximately 1500°C.
3. Drawing the molten glass onto the tin bath:
The molten glass is “floated” onto a bath of molten tin at a temperature of about 1000°C. It forms a ribbon with a working width of 3210mm which is normally between 3 and 25mm thick. The glass which is highly viscous and the tin which is very fluid do not mix and the contact surface between these two materials is perfectly flat.
4. Cooling of the molten glass in the annealing lehr:
On leaving the bath of molten tin, the glass – now at a temperature of 600°C – has cooled down sufficiently to pass to an annealing chamber called a lehr. The glass is now hard enough to pass over rollers and is annealed, which modifies the internal stresses enabling it to be cut and worked in a predictable way and ensuring flatness of the glass. As both surfaces are fire finished, they need no grinding or polishing.
5. Quality checks, automatic cutting, and storage:
After cooling, the glass undergoes rigorous quality checks and is washed. It is then cut into sheets of sizes of up to 6000mm x 3210mm which are in turn stacked, stored and ready for transport.
COMPOSITION OF GLASS
Nearly all commercial glasses fall into one of six basic categories or types. These categories are based on chemical composition. Within each type, except for fused silica, there are numerous distinct compositions.
- Soda-lime glass is the most common (90% of glass made), and least expensive form of glass. It usually contains 60-75% silica, 12-18% soda, 5-12% lime. Resistance to high temperatures and sudden changes of temperature are not good and resistance to corrosive chemicals is only fair.
- Lead glass has a high percentage of lead oxide (at least 20% of the batch). It is relatively soft, and its refractive index gives a brilliance that may be exploited by cutting. It is somewhat more expensive than soda-lime glass and is favored for electrical applications because of its excellent electrical insulating properties.
- Borosilicate glass is any silicate glass having at least 5% of boric oxide in its composition. It has high resistance to temperature change and chemical corrosion. Pipelines, light bulbs, photochromic glasses, sealed-beam headlights, laboratory ware, and bake ware are examples of borosilicate products.
- Aluminosilicate glass has aluminum oxide in its composition. It is similar to borosilicate glass but it has greater chemical durability and can withstand higher operating temperatures. Compared to borosilicate, aluminosilicates are more difficult to fabricate. When coated with an electrically conductive film, aluminosilicate glass is used as resistors for electronic circuitry.
- Ninety-six percent silica glass is a borosilicate glass, melted and formed by conventional means, then processed to remove almost all the non-silicate elements from the piece. By reheating to 1200°C the resulting pores are consolidated. This glass is resistant to heat shock up to 900°C.
- Fused silica glass is pure silicon dioxide in the non-crystalline state. It is very difficult to fabricate, so it is the most expensive of all glasses. It can sustain operating temperatures up to 1200°C for short periods.
HOW GLASS IS USED IN CONSTRUCTION

PROPERTIES OF GLASS
1. It absorbs, refracts or transmits light. It can be made transparent or translucent.
2. It can take excellent polish.
4. It is strong and brittle.
5. It can be blown, drawn or pressed.
6. It is not affected by atmosphere.
7. It has excellent resistance to chemicals.
8. It is available in various beautiful colours.
9. With the advancement in technology, it is possible to make glass lighter than cork or stronger than steel.
10. Glass panes can be cleaned easily.
VARIETIES OF GLASS
Float Glass:
Float glass is also called soda lime glass or clear glass. This is produced by annealing the molten glass and is clear and flat.
Tinted Glass:

Certain additions to the glass batch mix can add color to the clear glass without compromising its strength. Iron oxide is added to give glass a green tint; sulphar in different concentrations can make the glass yellow, red or black. Copper sulphate can turn it blue. etc.
Toughened Glass :

This type of glass is tempered, may have distortions and low visibility but it breaks into small dice-like pieces at modulus of rupture of 3600 psi. Hence it is used in making fire resistant doors etc. They are available in same weight and thickness range as float glass.
Laminated Glass:

This type of glass is made by sandwiching glass panels within a protective layer. It is heavier than normal glass and may cause optical distortions as well. It is tough and protects from UV radiation (99%) and insulates sound by 50%. Used in glass facades, aquariums, bridges, staircases, floor slabs, etc.
Shatterproof glass:

By adding a polyvinyl butyral layer, shatter proof glass is made. This type of glass does not from sharp edged pieces even when broken. Used in skylight, window, flooring, etc.
Extra clean glass:

This type of glass is hydrophilic i.e. The water moves over them without leaving any marks and photocatylitic i.e. they are covered with Nanoparticles that attack and break dirt making it easier to clean and maintain.
Double Glazed Units:
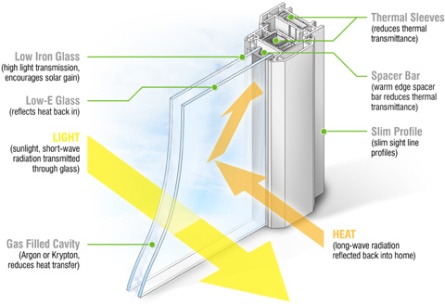
These are made by providing air gap between two glass panes in order to reduce the heat loss and gain. Normal glass can cause immense amount of heat gain and upto 30%of loss of heat of air conditioning energy. Green, energy efficient glass can reduce this impact.
Chromatic glass:

This type of glass can control daylight and transparency effectively. These glass are available in three forms- photochromatic (light sensitive lamination on glass), thermochromatic (heat sensitive lamination on glass) and electrochromatic (light sensitive glass the transparency of which can be controlled by electricity switch.) It can be used in meeting rooms and ICUs
Glass blocks:

Advantages:
1. Use of glass in construction work adds beauty to the building.
2. Its use fulfills the architectural view for external decoration.
3. By using glass in interior, it saves the space inside the building.
4. Glass cladding in building fulfill functional requirement of lighting, heat retention and energy saving.
5. Its use appear a sense of openness and harmonious.
6. As toughened glass is available, one can have good interior design with the use of glass in transparent staircase, colored shelves, ceiling etc.
7. Glass is an excellent material for thermal insulation, water proofing and energy conservation.
8. Glass is bad conductor of heat; it saves energy in air conditioning of building.
9. For making glass partition on upper floors, no extra design is required for slab as glass is light in weight.
Disadvantages:
1. As glass is very costly material, it may increase the budgeted cost of construction work.
2. Use of glass also enhances the cost of security.
3. Its use in hilly area and desert may cause more maintenance cost.
4. Glass is also unsafe for earthquake proven area.


Quiet useful, interesting and proudful
Keep updating….
LikeLiked by 1 person
Thank you!… I’ll be glad to see you again.
LikeLike